The Role of Thermodynamics in Everyday Life
Posted by Michaela Jackson on 24th May 2022
Updated 2023
Although the concept of “heat” is
commonplace, many people will struggle to define what “heat” actually is
without using the words “heat” or “hot”. Often the concept of “heat” is most
easily thought about in terms of a feeling or sensation, rather than a physical
transaction. Heat is much more than a feeling or a temperature - heat is the
transfer of energy. In fact, there is a whole sect of physics dedicated to the
study of heat as a form of moving energy called
thermodynamics. Heat is a form of energy transaction that surrounds
us every day, even when we may not realize it, and plays a large role in many
of the physical and chemical processes that shape our lives.

What is heat and how is it measured?
In order to understand heat, we first must clearly define it. Heat the transfer of thermal energy that is measurable through a unit of measurement called Joules (J) . This may be different from the definition you came up with. You may have thought to yourself, isn’t heat measured by temperature? The answer is no, not exactly. Although heat and temperature are closely related and are often used interchangeably in regular speech, they refer to different things.
Temperature is a physical property of an object and is measured a few different ways: degrees Fahrenheit (°F) mainly in the US and its territories, degrees Celsius (°C) by most other countries, and Kelvin (K) by the physics and chemistry communities. Temperature is a measurement which pinpoints the average kinetic energy the atoms of an object have. Kinetic energy refers to the energy of moving objects - in this case, those “moving objects” are the individual molecules and atoms making up one larger object. High temperatures (or “hotter” temperatures) mean high kinetic energy within the object, which means its molecules are moving around quickly. Adversely, low temperatures (or “cooler” temperatures) mean low kinetic energy within the object, which means its molecules are moving around much slower.
Again, heat is the actual transfer of thermal energy between the atoms and molecules of different objects - therefore, the study of heat and thermodynamics is actually the study of the behavior and changes in these atoms and molecules as energy flows between them ( source). Unlike temperature, heat is not something that an object “has” since it is not a property of an object. Instead heat is something that objects can gain or lose, as it is the movement of thermal energy, not the possession of thermal energy or lack thereof ( source).
An important concept to understand about heat energy is that heat always moves from high temperatures to low temperatures . As the temperature of an object increases, its molecules increase in size and begin to move much more quickly. This energy is eventually released, and heat continuously moves from the object with the higher temperature to the object with the lower temperature until the two objects reach the same temperature and achieve thermal equilibrium. A great example of this concept which you might relate to is trying to cool down a hot drink. To do this you may add some kind of other, colder liquid to the drink, or simply put ice cubes into the hot drink. Either way, when these cooler objects are added to the hot liquid, the original hot liquid begins to “cool down” and loses its heat energy to the cooler objects ( source). Eventually the drink and the milk or ice cubes will reach the same temperature, thus stopping the flow of energy. The milk mixes into the drink, or the ice cubes melt into liquid.

How is heat energy produced?
There are several ways to produce heat. One of the most commonly known methods is fire. The process of burning an object causes the chemical composition of that object to change into a different chemical composition. This chemical change causes energy to be released during the transformation. Even though many might believe burning to be a physical reaction as opposed to a chemical reaction, the opposite is true - the process of burning an object is a chemical reaction because a new substance is formed from the old, and comes with new chemical properties. This change cannot be reversed. For example, take the burning of natural gas, which is commonly used to heat homes. The main component of natural gas is methane, and when it is burned in home furnaces it combines with oxygen in the air and produces completely different chemicals, including carbon dioxide and water vapor ( source). The chemical changes induced by burning an object create heat as a byproduct of their reaction.
Another widely known method of producing heat is friction. Have you ever instinctually rubbed your hands together when it’s cold outside to warm them up? The new warmth is due to friction - the rubbing of two surfaces together, which makes the atoms and molecules of both surfaces move around more quickly, which increases their energy and heats up the areas experiencing friction ( source). Friction can even lead to burning, such as when one lights a match using the rough surface of the matchbox.

A staple in modern society is electricity. It powers our lives every day, but most people don’t think about the fact that electricity is another common producer of heat. In fact, the electric components in our laptops and computers produce so much heat that they are now fitted with miniature fans inside the system to help remove some of the thermal energy produced by the parts of the computer. Even something as simple as a light bulb is easy proof of electricity producing thermal energy. If you leave a lightbulb on for a long time continuously, then go to turn it off and remove it from the fixture, you will discover that the glass of the bulb itself is scalding hot! That is one reason why electricians often have to protect themselves from electrocution with protective gear such as insulating gloves and sleeves, safety boots, and flame-resistant clothing ( source).
The Sun is also a producer of a type of thermal energy called solar radiation , and is a form of heat we can experience every day. What makes the Sun unique from other heat production is that it requires absolutely no outside forces or human interaction to produce its heat. The Sun’s thermal radiation heats the earth due to its own extremely hot gasses, and is the only form of heat transfer that does not require a material to transmit its heat ( source). The Sun’s rays are powerful, so it is always important to wear adequate sunscreen, even if you think you may not need it. At best solar radiation can cause sunburn (which can easily become very severe, particularly for fair-skinned individuals), at the worst it can cause skin cancer.
How does heat transfer?
Just as there are several ways to produce heat, there are several ways to transfer heat as well. The three main ways that heat is transferred is through conduction, convection, and radiation. Thermal energy is transferred between two systems based on the difference in temperature between them. Remember, heat always flows from higher temperature objects to lower temperature objects until a temperature equilibrium is reached.
Conduction is an easily understandable way to transfer heat, because there are many examples we can find in everyday life. Conduction is the transfer of thermal energy through the molecules that make up an object . Based on the material of the object, heat can either be easily and efficiently transferred throughout the object’s molecules (then that object is considered a good conductor of heat), or not so easily transferred throughout the object’s molecules (that object is considered a poor conductor, or an insulator).
All materials have their own properties, including properties that can make them efficient conductors or efficient insulators. Examples of good conductors would be metals, such as gold, silver, iron, brass, bronze, copper, and aluminum, to name a few. In fact, copper and silver are known as the best heat conductors ( source). This is because the atoms in metals create a matrix formation that allows for their outer electrons to move around easily, unobstructed ( source). A common example of metals being an excellent conductor of heat is using a metal spoon to stir a pot of boiling water. You will notice how quickly the heat being experienced by one end of the spoon spreads to the other end where you are holding it, thus burning your fingers.
Examples of insulators (poor conductors) would be materials such as glass, wood, plastic, and even water. The worst conductor of heat is actually any type of gas - including the air around us, which is a mixture of many types of gasses ( source). These materials have atoms whose electrons are not as freely movable, thus preventing these materials from quickly transferring heat. Think of how long it takes for the sun to heat up water in a pool (I’m sure you’ve stepped into a surprisingly cold pool before, even though the sun has been up for hours), and how slowly the water loses the heat it has gained when the sun goes down.
Another way thermal energy is transferred is through a process known as convection, which only can occur in liquids and gasses. Convection refers to the heat transfer of fluids (both liquids and gasses) and occurs when a fluid is heated and carries that thermal energy with it, even when moving away from the heat source. The heating of the molecules in fluids causes those molecules closest to the heat source to expand and displace the rest of the fluid. The hottest molecules rise, pushing colder, more dense molecules downwards. This creates what is known as a convection current and is a continuous cycle of convection until the heat source is removed and all molecules return to an equilibrium temperature ( source).
A practical example of the convection process is, again, the pot of boiling water. At the bottom of the pot, closest to the heat source of the stove, the water particles in that area are heated up. As those particles expand they rise to the surface and push down the cooler particles until they heat up enough to rise themselves and cause more displacement. This is a common, everyday convection current that is both observable and useful.
Finally, thermal energy is also transferred through radiation, which can occur in any transparent medium whether solid or fluid. The emission of electromagnetic waves creates thermal radiation, carrying thermal energy away from the emitting object (source). All materials radiate thermal energy on some level based on their temperature - the higher the temperature of the material, the more movement in its atoms and molecules, the more thermal energy that will be radiated ( source).
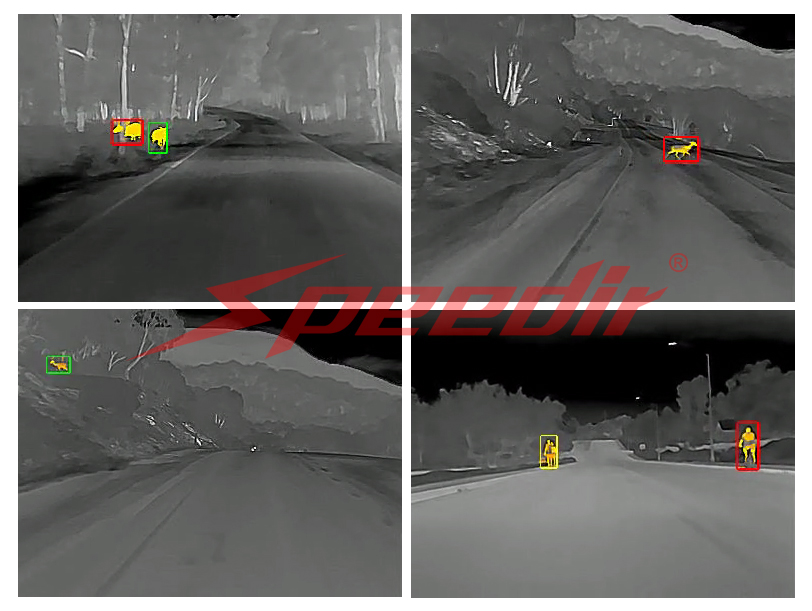
It is because of thermal radiation that technology like infrared cameras, which use infrared sensors to detect the heat signatures of objects in the viewfinder, are so useful. Everything generates some type of heat signature - from the Sun to a human to a rabbit. The heat signature comes from thermal radiation of infrared waves, a type of electromagnetic wave on the electromagnetic scale between visible light and radio waves. Those infrared waves are detectable to infrared sensors and cameras such as the Night Owl Thermal Night Vision Dashcam from Speedir, which helps increase driver safety at night by detecting potential hazards on the road through reading heat signatures.
The Laws of Thermodynamics
One cannot properly talk about heat and energy transfer without mentioning the Laws of Thermodynamics. There are four total laws, numbering from zero to three, and they establish the most basic rules of heat energy transfer in terms of physics ( source):
●First Law of Thermodynamics (the law of conservation of energy): “Energy can neither be created nor be destroyed, it can only be transferred from one form to another.”
○Translation: “Energy can only be transferred, and the amount of energy in the universe will remain constant.”
●Second Law of Thermodynamics (the law of increased energy): “The entropy (a quantitative index that describes the quality of energy through the randomness and disorder of a system’s particles - source) of any isolated system always increases. Any isolated system spontaneously evolves towards thermal equilibrium - the state of maximum entropy of the system.”
○Translation: “When using or transferring energy, there will always be some energy leftover that is unusable and must be discarded via a waste transferring process.”
●Third Law of Thermodynamics: “The entropy of a system approaches a constant value as the temperature approaches absolute zero.”
○Translation: “The lower the temperature of a system, the less entropy (or random movement) its particles have. As a system’s temperature approaches absolute zero, so does its entropy.”
●Zeroth Law of Thermodynamics: “If two thermodynamic systems are in thermal equilibrium with a third system separately, then they are in thermal equilibrium with each other.”
○Translation: “If two separate systems are in thermal equilibrium with a separate third system, that means the two original systems must also be in equilibrium with each other, because they are all three at the same equilibrium.”
Confusing? Yes. Anyone who is not in the scientific arena might find these concepts to be a little abstract. Your main takeaway from the laws of thermodynamics should be this: the amount of energy in the universe will always be the same but can change forms or transfer between systems; no use of energy is 100% efficient; the closer a system gets to absolute zero the less its particles move and the less heat energy it has; finally, if A = C and B = C then also A = B (Let A be system 1, B be system 2, and C be system 3).
Thermodynamics in the Real World
One of the most unique and interesting ways thermodynamics is used in real world applications is through infrared detection systems such as infrared cameras. As previously mentioned, infrared cameras use thermal radiation (AKA infrared waves) to detect heat signatures. The development of thermal cameras has been a great advancement for research and safety in many fields - from physics to chemistry, from military to commercial, from security to individual.
How can thermodynamics help you? The best way to get started with using infrared technology to enhance your life and safety is through infrared dashcams.
Driving is already dangerous on its own if you think about it - you’re hurtling a large metal beast down highways up to 75-80 mph. What makes driving the most dangerous is factors you cannot control, such as wildlife in the road, pedestrians, other vehicles, and more. Particularly at nighttime it becomes very difficult to detect road hazards like deer or bikers even with your brights on, and even then you have very little time to react if need be.
That is where thermal dashcams come in! Enter Speedir, a leader in the vehicle safety accessories industry and an innovator when it comes to using advanced infrared and AI technology to make state-of-the-art infrared thermal dashcams affordable for everyone.
Speedir offers two models of infrared dashcams: the Night Owl thermal night vision camera , and the Night Owl Plus infrared deer detector. Both cameras use the best in infrared and thermal technology to detect heat signatures of road hazards, and are much more accurate than a normal “night vision” camera or using brights because it requires no light at all to precisely detect hazardous heat signatures. However, if you’re looking for that extra mile, the Night Owl Plus also offers AI detection and verbal warnings when heat signatures are detected, allowing a driver to have maximum concentration and safety on the road. Click the above links to learn more.
Sources:
- https://wonders.physics.wisc.edu/what-is-heat/
- https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/2.9/primary/lesson/chemical-change-ms-ps/
- https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/10.4/primary/lesson/friction-ms-ps/
- https://www.solarschools.net/knowledge-bank/energy/types/thermal
- https://itap.edu/choosing-the-best-personal-protective-equipment-for-electricians/
- https://www.sciencedirect.com/book/9780123749796/introduction-to-biomedical-engineering
- https://energyeducation.ca/encyclopedia/Heat_vs_temperature
- https://k8schoollessons.com/conductors-and-insulators/
- https://byjus.com/questions/why-are-metals-good-conductors-of-electricity/
- https://www.universetoday.com/82331/what-is-conduction/
- https://www.machinedesign.com/learning-resources/whats-the-difference-between/document/21834474/whats-the-difference-between-conduction-convection-and-radiation
- https://byjus.com/physics/thermodynamics/#laws-of-thermodynamics
- https://byjus.com/jee/second-law-of-thermodynamics/

